- GPS Cancer Adoption by Oncologists Increased in Q4: 264 Oncologists Ordered 1,286 GPS Commercial & Research Tests YTD, with 452 tests in Q4, of which 326 Commercial and 126 Research
- First Consecutive 100 GPS Cancer Tests Completed at a Single Academic Institution (Feb-Nov 2016) Demonstrating Significant Increase in Identification of an FDA Approved Drug or Clinical Trial for Patients with Refractory Cancer
- Expanded Adoption of GPS Cancer in Florida with Genomics Laboratory Licensing by the State of Florida in Q4 with 138 tests ordered in 2016
- GPS Cancer Payor Coverage: Total of Eight Payer Contracts with Self-Employed and Payors Now Cover GPS Cancer; Potential Sales Pipeline of GPS Cancer Payors Increased to 27 in Q4 from 18 in Q3
- International Association of Fire Fighters (IAFF) endorses GPS Cancer as a benefit for approximately 310,000 IAFF employee members and approximately 300,000 beneficiaries across the US and Canada
- Pilot Trial with Horizon Blue Cross Blue Shield New Jersey for GPS Cancer Initiated
- Discussions Underway with CMS for Both Potential Local Coverage Determination (LCD) and National Coverage for GPS Cancer
- NantOS, Device Connect and NaviNet: Completed 39 Go Live Projects; Number 2 Market Share in Connected Care with over 25,000 Device Connects Licenses Sold to Date with 18 EMRs Integrated Across more than 300 Provider Client Sites
- Increases NantOS and NaviNet contractual commitments from 35 in Q3 to 96 in Q4
- Full Year 2016 Total Net Revenue Increased 72% to $100 Million from $58 Million in 2015 With SaaS Revenue Increasing More Than 181%
CULVER CITY, Calif.–(BUSINESS WIRE)–Mar. 30, 2017– NantHealth, Inc. (NASDAQ-GS: NH), a next-generation, evidence-based, personalized healthcare company, today reported financial results for its fourth quarter and full year ended December 31, 2016. The company began operating as a publicly traded entity in June 2016 when it launched GPS Cancer as a commercial product.
GPS Cancer – Highlights
- First Consecutive 100 GPS Cancer Tests (Feb-Nov 2016) Completed in a Single Academic InstitutionDemonstrating Significant Increase Identification of an FDA Approved Drug or Clinical Trial for Patients with Refractory Cancer
- Number of covered cancer lives: at December 31, 2016, the number of patients with cancer covered by a payer for GPS testing was approximately 322,000 including lives anticipated upon completion of a pilot project with Horizon Blue Cross Blue Shield
- Number of GPS Cancer payers: at December 31, 2016 the number of payers covering GPS Cancer was eight. Discussions are in progress with 27 payers, which includes self-insured employers and insurers, increasing from 18 in Q3
- Potential Local Coverage Determination (LCD) & Potential National Coverage by CMS: Discussions are underway to potentially provide both local coverage and national coverage with CMS and FDA regarding GPS Cancer coverage by Medicare
- Number of GPS Cancer tests: 1,286 GPS Commercial & Research Tests YTD with 452 ordered in Q4; 233 commercial tests delivered in Q4, up from 154 in Q3. Number of Oncologists Ordering GPS Cancer Increased to 264 in Q4 from 181 in Q3
- Expanded adoption of GPS Cancer in Florida with the new Genomics Lab licensing agreement with the state
- The International Association of Fire Fighters (IAFF) has endorsed GPS Cancer, as a valuable benefit that will be accessible for approximately 310,000 IAFF members and 300,000 beneficiaries across the US and Canada
- 87 GPS Cancer tests were ordered under an agreement with a pediatric cancer research institution in California
“Our GPS Cancer Test continues to gain coverage in both the commercial health insurance and self-employed payor markets, and we are seeking to build adoption for GPS Cancer among oncologists through our ongoing efforts to educate the medical community about the test’s value,” said Patrick Soon-Shiong, M.D., Chief Executive Officer and Chairman of NantHealth.
GPS Cancer has shown successful adoption in the research setting. Under CLIA-CAP conditions, NantHealth’s partner NantOmics has completed the analysis of thousands of patient samples with GPS demonstrating the efficacy or resistance thresholds of chemotherapy and targeted therapy agents in cancer treatment. The significance of these validated findings is the ability to better inform the practicing oncologist of the standard of care which would most likely benefit the patient before treatment begins. Over the last six months, a concerted educational process has been undertaken to demonstrate the clinical utility of GPS Cancer for patients receiving chemotherapy, hormonal therapy, targeted therapy and monoclonal antibody therapy. GPS Cancer is the only comprehensive test covering whole genome tumor/normal sequencing and also transcriptomics and quantitative proteomics. By addressing proteomics at the specific pathway level related to the mechanism of action of the therapeutics, GPS Cancer has the potential to provide actionable information at the point of care.
The unique proteomic profiling within the GPS Cancer test covers a multitude of resistance and sensitivities biomarkers. For example, we have tested 1,709 GPS samples for ERCC1 and that measuring this protein expression can predict the response to platinum based chemotherapy. For example, ERCC1 is found in patients with non-small cell lung cancer (NSCLC). The GPS test can show the exact level of ERCC1 expression in patients and identify those with resistance levels of 75 amol per microgram or above. Research studies show that oxaliplatin, cisplatin, and carboplatin resistance levels are found to be at 75 amol per microgram in which 594 of the 1,709 samples were above the cutoff showing resistance to the drug where it should not be given, helping the patient avoid unnecessary side effects of the drug.
Potential Local Coverage Determination (LCD) and National Coverage for GPS Cancer with CMS
NantHealth continues to make positive advances in discussions with CMS (Centers for Medicare Medicaid Services) and representatives from the MolDX Program for GPS Cancer. In March 2017, NantHealth presented at CMS for both local and national determination on clinical experience with GPS Cancer. The recent positive coverage determination by Palmetto GBA for comprehensive genomic profiling in Non-Small Cell Lung Cancer (NSCLC) is encouraging, and the company believes that the GPS Cancer test can meet the eligibility requirements for payment once NantHealth is in the position to submit claims under this LCD.
NantOS – Highlights
During the fourth quarter, the company:
- Completed 19 go-live projects across NantOS and device connects
- Completed 20 go-live projects, of which 19 were on NaviNet and one on NaviNet Document Exchange
- Increased, renewed or expanded contractual commitment to 96 current clients from 35 at the end of Q3
Dr. Soon-Shiong added, “Our full year net revenue increased 72%, largely due to growth in SaaS revenue of more than 181%. Our total bookings in total contract value, which includes net new sales, renewals and product expansions, were approximately $28 million in fourth quarter and $74 million for the year. Overall, we finished the year well ahead of plan on this metric.”
Subsequent to the year end, the company exhibited its new suite of oncology solutions and provided demos at the Interoperability Showcase floor, during the HIMSS 2017 Annual Conference & Exhibition, which is recognized as one of the premier healthcare industry conferences, HIMSS17. Independent market research and opinion research company, Black Book Research, awarded the company the honor of #1 in Personalized Healthcare Solutions, Precision Medicine Tools and for achieving Highest Client Satisfaction.
Financial Highlights
For the 2016 full year, total net revenue increased 72% to $100.4 million from $58.3 million in 2015. Gross profit rose 19% to $28.0 million from $23.5 million for 2015. Selling, general and administrative (SG&A) expenses were $120.7 million compared with $69.0 million for the prior year. Research and development (R&D) expenses increased to $61.6 million from $23.8 million last year. For the full year 2016, the company recorded loss from related party equity method investment of $41.0 million. This includes a $29.8 million impairment charge related to the company’s investment in NantOmics, which was driven by the impact of the delay in the sales growth of the GPS test compared with the company’s expectations at the time of its investment in NantOmics. Net loss for 2016 was $184.1 million, or $1.69 per share, compared with $72.0 million, or $0.99 per share, for 2015. We expect to continue to incur operating losses over the near term as we drive adoption of GPS Cancer, expand our commercial operations, and invest further in NantHealth solutions.
Financial results for 2016 included approximately $54.0 million in non-cash, stock-based compensation expense, $41.0 million loss from related party equity method investment, and $22.8 million of intangible amortization, equal to $1.05 per share in total, offset by $23.3 million net income tax benefit adjustments, equal to $0.21 per share. On a non-GAAP basis, for 2016, adjusted net loss was $81.0 million, or $0.69 per share, compared with $53.6 million, or $0.54 per share, in the prior year.
For the 2016 fourth quarter, total net revenue increased 18% to $24.1 million from $20.4 million in last year’s fourth quarter. Gross profit was $4.3 million compared with $9.6 million, for the 2015 fourth quarter. SG&A expenses were $21.3 million compared with $16.6 million for the prior year fourth quarter. R&D expenses increased to $12.8 million from $7.2 million in the comparable quarter of last year. For the fourth quarter of 2016, the company recorded loss from related party equity method investment of $33.1 million, which includes the $29.8 million impairment charge mentioned above. Net loss for the 2016 fourth quarter was $60.0 million, or $0.49 per share, compared with $17.9 million, or $0.35 per share, for the 2015 fourth quarter.
Financial results for the 2016 fourth quarter included approximately $5.5 million of intangible amortization and $5.0 million in non-cash, stock-based compensation expense, equal to $0.09 per share in total, offset by $4.5 million net income tax benefit adjustment, equal to $0.04 per share. On a non-GAAP basis, for the 2016 fourth quarter, adjusted net loss was $21.6 million, or $0.18 per share, compared with $10.9 million, or $0.10 per share, in the prior year.
In December 2016, we issued convertible notes to a related party and others for net proceeds of $9.9 million and $92.8 million, respectively, after deducting underwriting discounts and commissions and other offering costs of $4.3 million.
Other Corporate Highlights
- In December, together with NantKwest and NantOmics, we announced the public availability of QUILT Programs (Quantum Immuno-oncology Lifelong Trial), aimed to harness and orchestrate all elements of the immune system through the testing of novel treatment combinations, within the national clinical trials database, www.ClinicalTrials.gov. QUILT utilizes GPS Cancer – a unique, comprehensive set of tests integrating quantitative proteomics, and whole genome (DNA) and transcriptome (RNA) sequencing—to provide oncologists with a full molecular profile of each patient’s cancer not only for an initial biopsy before the trial begins, but throughout the trial process to fully understand how each therapy impacts the patient’s tumor activity.
- In December, we entered into an exclusive reseller agreement for GPS Cancer advanced molecular analysis with Lunatus, a company focused on linking international healthcare companies and regional healthcare professionals in Arabian Gulf and Middle East markets.
- In November, we entered into an exclusive reseller agreement for GPS Cancer tests with Sorgente, the market leader in biobank, stem cell and now molecular profiling testing in Italy.
Conference Call Information and Forward-Looking Statements
Later today, the company will host a conference call at 1:30 p.m. PT (4:30 p.m. ET) to review its results of operations for the fourth quarter and full year ended December 31, 2016. The conference call will be available to interested parties by dialing 844-309-3709 from the U.S. or Canada, or 281-962-4864 from international locations, passcode 92890709. The call will be broadcast via the Internet at www.nanthealth.com. Listeners are encouraged to visit the website at least 10 minutes prior to the start of the scheduled presentation to register, download and install any necessary audio software. A playback of the call will be archived and accessible on the same website for at least three months.
Discussion during the conference call may include forward-looking statements regarding such topics as, but not limited to, the company’s financial status and performance, regulatory and operational developments, and any comments the company may make about its future plans or prospects in response to questions from participants on the conference call.
Use of Non-GAAP Financial Measures
This news release contains references to Non-GAAP financial measures, including adjusted net loss and adjusted net loss per share, which are financial measures that are not prepared in conformity with United States generally accepted accounting principles (U.S. GAAP). The Company’s management believes that the presentation of Non-GAAP financial measures provides useful supplementary information regarding operational performance, because it enhances an investor’s overall understanding of the financial results for the Company’s core business. Additionally, it provides a basis for the comparison of the financial results for the Company’s core business between current, past and future periods. Other companies may define these measures in different ways. Non-GAAP financial measures should be considered only as a supplement to, and not as a substitute for or as a superior measure to, financial measures prepared in accordance with U.S. GAAP. Non-GAAP per share numbers are calculated based on one class of common stock and do not incorporate the effects, if any, of using the two-class method.
About NantHealth, Inc.
NantHealth, Inc., a member of the NantWorks ecosystem of companies, is a next-generation, evidence-based, personalized healthcare company enabling improved patient outcomes and more effective treatment decisions for critical illnesses. NantHealth’s unique systems-based approach to personalized healthcare applies novel diagnostics tailored to the specific molecular profiles of patient tissues and integrates this molecular data in a clinical setting with large-scale, real-time biometric signal and phenotypic data to track patient outcomes and deliver precision medicine. For nearly a decade, NantHealth has developed an adaptive learning system, which includes its unique software, middleware and hardware systems infrastructure that collects, indexes, analyzes and interprets billions of molecular, clinical, operational and financial data points derived from novel and traditional sources, continuously improves decision-making and further optimizes our clinical pathways and decision algorithms over time. For more information please visit www.nanthealth.com.
About GPS Cancer™
GPS Cancer™ is a unique, comprehensive test available through NantHealth. GPS Coverage
GPS Cancer integrates whole genome (DNA) sequencing, whole transcriptome (RNA) sequencing, and quantitative proteomics through mass spectrometry, providing oncologists with a comprehensive molecular profile of a patient’s cancer to inform personalized treatment strategies. GPS Cancer testing is conducted in CLIA-certified and CAP-accredited laboratories, and is a key enabler for Cancer Breakthroughs 2020, the world’s most comprehensive cancer collaborative initiative seeking to accelerate the potential of combination immunotherapy as the next generation standard of care in cancer patients.
This news release contains certain statements of a forward-looking nature relating to future events or future business performance. Forward-looking statements can be identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates,” “plans,” “will,” “outlook” and similar expressions. Forward-looking statements in this news release include, but are not limited to, statements about: our ability to increase adoption of and the commercial success of GPS Cancer and our other solutions; and our plans or ability to obtain reimbursement for GPS Cancer, including third-party payors, such as commercial insurance companies and health maintenance organizations, and government insurance programs, such as Medicare and Medicaid. Risks and uncertainties include, but are not limited to: our ability to successfully integrate a complex learning system to address a wide range of healthcare issues; our ability to successfully amass the requisite data to achieve maximum network effects; appropriately allocating financial and human resources across a broad array of product and service offerings; raising additional capital as necessary to fund our operations; achieving significant commercial market acceptance for our sequencing and molecular analysis solutions; establish relationships with, key thought leaders or payors’ key decision makers in order to establish GPS Cancer as a standard of care for patients with cancer; our ability to grow the market for our Systems Infrastructure, NantOS and NantOS apps; successfully enhancing our Systems Infrastructure, NantOS or NantOS apps to achieve market acceptance and keep pace with technological developments; customer concentration; competition; security breaches; bandwidth limitations; our ability to continue our relationship with NantOmics; our ability to obtain regulatory approvals; dependence upon senior management; the need to comply with and meet applicable laws and regulations; and unexpected adverse events. Forward-looking statements are based on management’s current plans, estimates, assumptions and projections, and speak only as of the date they are made. We undertake no obligation to update any forward-looking statement in light of new information or future events, except as otherwise required by law. Forward-looking statements involve inherent risks and uncertainties, most of which are difficult to predict and are generally beyond our control. Actual results or outcomes may differ materially from those implied by the forward-looking statements as a result of the impact of a number of factors, many of which are discussed in more detail in our reports filed with the Securities and Exchange Commission.
FINANCIAL TABLES FOLLOW
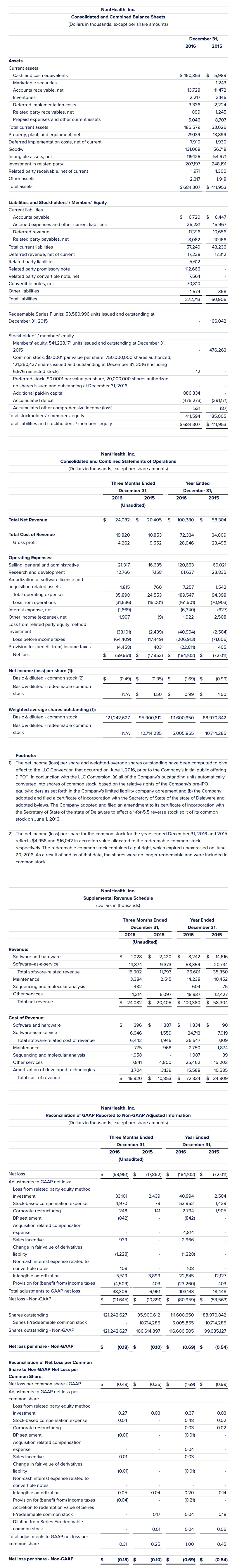
1) The net loss per share – non-GAAP, weighted-average shares outstanding, weighted average Series F units/redeemable stock and shares outstanding – non-GAAP, have been computed to give effect to the LLC conversion that occurred June 1, 2016 prior to our initial public offering. In conjunction with the LLC Conversion, (a) all of our outstanding units automatically converted into shares of common stock, based on the relative rights of our pre-IPO equityholders as set forth in the limited liability company agreement and (b) we adopted and filed a certificate of incorporation with the Secretary of State of the state of Delaware and adopted bylaws. We filed an amended certificate of incorporation to effect a 1-for-5.5 reverse stock split of our common stock on June 1, 2016.
2) The weighted-average shares outstanding have been further adjusted to account for the redeemable Series F units (converted to common stock in conjunction with the LLC conversion), whose Put Right expired on June 20, 2016. Prior to June 20, 2016, these units/shares of common stock were classified as redeemable members’/stockholders’ equity in the balance sheet, and as such, were not included in the weighted-average shares outstanding prior to June 20, 2016. The Put Right expired June 20, 2016, and the shares were no longer redeemable and are included in shareholders’ equity as of December 31, 2016. The weighted-average shares are adjusted to include the redeemable common stock in the weighted-average shares outstanding for the entire period.
3) Net loss – Non-GAAP excludes, among others, the effects of (1) loss from related party equity method investment, (2) stock based compensation expense, (3) intangible amortization, (4) corporate restructuring expenses, (5) acquisition related compensation expense, (6) BP settlement other income, (7) acquisition-related sales incentives, which have been recorded as contra revenue, (8) change in fair value of derivatives liability and (9) non-cash interest expense related to convertible notes. Provision for (benefit from) income taxes impact of the conversion from a limited liability corporation to a corporation, the impact of convertible notes offering and the impact of intangibles amortization. Adjusted shares outstanding include Series F redeemable shares as if converted to common shares on January 1, 2015.
View source version on businesswire.com: http://www.businesswire.com/news/home/20170330006213/en/
Investor Contact:
Robert Jaffe
[email protected]
424.288.4098



