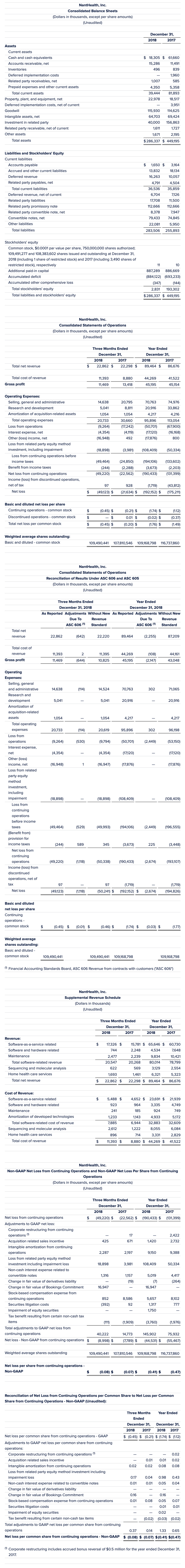
- Total Revenue in Q4 was $22.9 Million
- SaaS revenue of $17.3 million in Q4, up 9% from Q3
- Enhanced efficiencies continued to drive significantly lower operating expenses in both Q4 and full year of 2018
- For 2018, substantially improved adjusted Non-GAAP bottom line results
- Sequencing and Molecular Analysis, 1,021 total tests ordered in Q4, including 539 GPS Cancer® and 482 Liquid GPS℠ Tests; continued sequential quarterly growth in total tests ordered
CULVER CITY, Calif.–(BUSINESS WIRE)–Mar. 28, 2019– NantHealth, Inc. (NASDAQ-GS: NH), a next-generation, evidence-based, personalized healthcare company, today reported financial results for its fourth quarter ended December 31, 2018.
“Our financial results over 2018 reflect continued overall progress to our business,” said Bob Petrou, Interim Chief Financial Officer of NantHealth. “On a sequential quarterly basis, we grew revenue, significantly lowered total operating expenses and reduced our cash burn. We are particularly pleased with the growth of our entire SaaS business. Looking ahead, our well-developed sales pipeline bodes well for the company’s topline, and our entire team is focused on further growing the business, managing costs and driving meaningful improvement in our financial performance.”
Software and Services Highlights:
- Clinical Decision Support (Eviti):
- In Q4, deployed release 7.5, with new features that include:
- A new configuration, known as the Treatment Warning and Deviation Configuration, that enables payers to display custom drug deviation messages, enhancing value-based care and compliance with payer guidelines
- A new analyzer deviation that supports the Febrile Neutropenia (FN) risk factor associated with white blood cell (WBC) growth factors, a key driver of regimen costs
- Updated the Medical Policy QA report that assists partners/resellers with their internal quality assurance process
- Enhanced the Precision Insights Portal, features include:
- All treatment plans that are presented to a user are saved and stored, enabling analytics for the understanding of treatment plan trends and improvement in the presentation of the plans to users
- Enhanced drug mapping management feature in the portal enables a comparison of the Precision Insights portal recommended drugs to the evidence based Eviti drug recommendation to help align to the standard of care library
- In Q4, deployed release 7.5, with new features that include:
- Payer Engagement (NaviNet):
- Completed 12 client service implementation projects in Q4, for a total of 57 in 2018
- In Q4, introduced two key features for NaviNet Open
- Routing Attributes for Document Exchange were enhanced to provide health plans more control over document routing to users
- NaviNet Open subscribers are now provided a detailed view of user activity for all Authorization Appeals in a monthly report delivered to the health plan
- In December 2018, at the Healthcare Payers Transformation Assembly hosted by the Millennium Alliance, the company met with Payer groups and led a roundtable discussion entitled, “Looking at the Challenges Health Plans Face with the Convergence of Precision Medicine and Value Based Care.” As a result, the company is engaged in a number of ongoing sales conversations based on discussions initiated at this event
- Connected Care (DeviceConX):
- In Q4, completed a DeviceConX implementation in Sweden, which included our first deployment with physiological waveform capabilities
- In Q4, as previously announced, collaborated with B. Braun Australia, GE Healthcare and iProcedures to demonstrate the exchange of data between patient devices and medical records at the Interoperability Showcase™ as part of the Healthcare Information and Management Systems Society (HIMSS) Asia Pacific Conference
- Significantly increased connectivity license sales in Q1, driving improved recurring maintenance revenue on a go forward basis
- In Q1, deployed DeviceConX Version 5.15 upgrade, with the ability to push OS security patches directly to HBox Connected Care hardware devices
Sequencing and Molecular Analysis – Highlights
- In Q4, total orders (GPS Cancer and Liquid GPS) increased 10% to 1,021 from 930 in Q3
- In Q4, the company expanded the molecular analysis reporting of GPS Cancer to provide information on a patient’s pharmacogenomic profile. These results, now available in the GPS Cancer test report, provide insights into potential drug toxicity and/or interactions
- In December 2018, at the San Antonio Breast Cancer Symposium, the company and NantOmics presented the findings of three investigations, which examine the theme of providing oncologists with insights that enable cancer treatment tailored to the individual characteristics of each patient
- In Q4, expanded existing employer reimbursement contract with Northwest Firefighters Benefits Trust to include Liquid GPS coverage and signed a net new reimbursement contract for both GPS Cancer and Liquid GPS with an additional city fire/police employee trust
Business and Financial Highlights
The company adopted a new revenue recognition standard on January 1, 2018. Please note that the financial results presented below include only new revenue standard values. For additional information and reconciliations of the company’s financial results between the new and previous revenue recognition standard, see the additional tables included in this press release and in the company’s Form 10-K to be filed with the Securities and Exchange Commission.
For the 2018 full year, total net revenue increased 3.2% to $89.5 million from $86.7 million in 2017. Gross profit was $45.2 million, or 51% of total net revenue, compared with $45.2 million, or 52% of total net revenue, for the prior year. Selling, general and administrative (SG&A) expenses decreased to $70.8 million from $75.0 million in 2017. Research and development (R&D) expenses decreased to $20.9 million from $33.9 million in 2017.
Financial results for 2018 included a non-cash charge for loss from related party equity method investment, including impairment, of $108.4 million, and an unrealized loss from change in fair value of the Bookings Commitment liability of $16.9 million. Net loss from continuing operations, net of tax, was $190.4 million, or $1.74 per share, compared with $131.4 million, or $1.12 per share, for the 2017 full year. Loss from discontinued operations, net of tax, was $1.7 million, or $0.02 per share, compared with $43.8 million, or $0.37 per share. Net loss was $192.2 million, or $1.76 per share, compared with $175.2 million, or $1.49 per share, for 2017.
For the 2018 full year, on a non-GAAP basis, adjusted net loss from continuing operations was $44.5 million, or $0.41 per share, compared with $55.5 million, or $0.47 per share, for 2017.
For the 2018 fourth quarter, total net revenue was $22.9 million, compared with $22.3 million in 2017 fourth quarter. Gross profit was $11.5 million, or 50% of total net revenue, compared with $13.4 million, or 60% of total net revenue, for the prior year. SG&A expenses declined to $14.6 million, from $20.8 million in 2017 fourth quarter. R&D expenses decreased to $5.0 million from $8.8 million.
Financial results for the fourth quarter of 2018 included a non-cash charge for loss from related party equity method investment, including impairment, of $18.9 million, and an unrealized loss from change in fair value of the Bookings Commitment liability of $16.9 million. Net loss from continuing operations, net of tax, was $49.2 million, or $0.45 per share, compared with $22.6 million, or $0.21 per share, for the 2017 fourth quarter. Net loss was $49.1 million, or $0.45 per share, compared with $21.6 million, or $0.20 per share, for 2017 fourth quarter.
For the 2018 fourth quarter, on a non-GAAP basis, adjusted net loss from continuing operations was $9.0 million, or $0.08 per share, compared with $7.8 million, or $0.07 per share, for the 2017 fourth quarter.
In August 2017, NantHealth sold its provider/patient engagement assets to Allscripts to focus on core competencies and accelerate the plan to achieve profitability. As a result, the company has classified the current and prior period operating results of its provider/patient engagement business as discontinued operations. All results presented above represent the company’s continuing operations.
Conference Call Information and Forward-Looking Statements
Later today, the company will host a conference call at 1:30 p.m. PT (4:30 p.m. ET) to review its results of operations for the fourth quarter ended December 31, 2018. The conference call will be available to interested parties by dialing 844-309-3709 from the U.S. or Canada, or 281-962-4864 from international locations, passcode 1059708. The call will be broadcast via the Internet at www.nanthealth.com. Listeners are encouraged to visit the website at least 10 minutes prior to the start of the scheduled presentation to register, download and install any necessary audio software. A playback of the call will be archived and accessible on the same website for at least three months.
Discussion during the conference call may include forward-looking statements regarding topics such as the company’s financial status and performance, regulatory and operational developments, and other comments the company may make about its future plans or prospects in response to questions from participants on the conference call.
Use of Non-GAAP Financial Measures
This news release contains references to Non-GAAP financial measures, including adjusted net loss and adjusted net loss per share, which are financial measures that are not prepared in conformity with United States generally accepted accounting principles (U.S. GAAP). The Company’s management believes that the presentation of Non-GAAP financial measures provides useful supplementary information regarding operational performance, because it enhances an investor’s overall understanding of the financial results for the Company’s core business. Additionally, it provides a basis for the comparison of the financial results for the Company’s core business between current, past and future periods. Other companies may define these measures in different ways. Non-GAAP financial measures should be considered only as a supplement to, and not as a substitute for or as a superior measure to, financial measures prepared in accordance with U.S. GAAP. Non-GAAP per share numbers are calculated based on one class of common stock and do not incorporate the effects, if any, of using the two-class method.
About NantHealth, Inc.
NantHealth, a member of the NantWorks ecosystem of companies, provides leading solutions across the continuum of care for physicians, payers, patients and biopharmaceutical organizations. NantHealth enables the use of cutting edge data and technology towards the goal of empowering clinical decision support and improving patient outcomes. NantHealth’s comprehensive product portfolio combines the latest technology in molecular analysis (GPS Cancer® and Liquid GPS℠) payer/provider platforms that exchange information in near-real time (NaviNet and Eviti), and connected care solutions that deliver Medical Device Interoperability (MDI). NantHealth’s GPS Cancer® molecular profiling provides comprehensive DNA & RNA tumor-normal profiling combined with pharmacogenomics analysis. Liquid GPS℠ provides non-invasive testing of cfDNA and cfRNA to monitor cancer mutations and potentially select targeted therapies, chemotherapies, and immunotherapies. For more information, please visit www.nanthealth.com.
About GPS Cancer®
GPS Cancer® is a unique, comprehensive test available through NantHealth. GPS Cancer integrates tumor/normal DNA and RNA sequencing, with enhanced expression analysis and bioinformatics of complex biologic pathway systems, providing oncologists with a comprehensive molecular profile of a patient’s cancer to inform personalized treatment strategies. GPS Cancer testing is conducted in CLIA-certified and CAP-accredited laboratories. For more information, visit https://www.nanthealth.com/gps-cancer/.
About Liquid GPS℠
Liquid GPS is a blood-based molecular test that provides oncologists with a powerful tool for noninvasive tumor profiling and quantitative monitoring of treatment response. Liquid GPS looks beyond cfDNA to cfRNA, which allows profiling and trending of actionable biomarkers that cannot be assessed through cfDNA alone. In addition to providing molecular insight into key guidelines-based biomarkers (e.g., EGFR, ALK, ROS1, KRAS), this powerful RNA-based approach enables a variety of capabilities and applications not typically available from a liquid biopsy test.
This news release contains certain statements of a forward-looking nature relating to future events or future business performance. Forward-looking statements can be identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates,” “plans,” “will,” “outlook” and similar expressions. Forward-looking statements are based on management’s current plans, estimates, assumptions and projections, and speak only as of the date they are made. Risks and uncertainties include, but are not limited to: our ability to successfully integrate a complex learning system to address a wide range of healthcare issues; our ability to successfully amass the requisite data to achieve maximum network effects; appropriately allocating financial and human resources across a broad array of product and service offerings; raising additional capital as necessary to fund our operations; achieving significant commercial market acceptance for our sequencing and molecular analysis solutions; establish relationships with, key thought leaders or payers’ key decision makers in order to establish GPS Cancer and Liquid GPS as a standard of care for patients with cancer; our ability to grow the market for our Systems Infrastructure, and applications; successfully enhancing our Systems Infrastructure and applications to achieve market acceptance and keep pace with technological developments; customer concentration; competition; security breaches; bandwidth limitations; our ability to continue our relationship with NantOmics; our ability to obtain regulatory approvals; dependence upon senior management; the need to comply with and meet applicable laws and regulations; unexpected adverse events; clinical adoption and market acceptance of GPS Cancer and Liquid GPS; and anticipated cost savings. We undertake no obligation to update any forward-looking statement in light of new information or future events, except as otherwise required by law. Forward-looking statements involve inherent risks and uncertainties, most of which are difficult to predict and are generally beyond our control. Actual results or outcomes may differ materially from those implied by the forward-looking statements as a result of the impact of a number of factors, many of which are discussed in more detail in our reports filed with the Securities and Exchange Commission.
FINANCIAL TABLES FOLLOW